Key role for ocean ecosystems in removing CO2
New NESSC-study underlines important role of surface region of ocean in dissolving calcium carbonates.
A tiny, but important chemical compound is found everywhere in the ocean: calcium carbonate. It forms the principal building block for many sea shells, corals and plankton. When calcium carbonate dissolves, it acts like an anti-acid pill: it removes carbon dioxide gas (CO2) from seawater, lessening the impact of human CO2 emissions on the global climate system. In a new study in Nature Geoscience, NESSC-researchers of Utrecht University show that calcium carbonate minerals largely dissolve at the surface region of the ocean due to biological recycling – or at the ocean floor after having sunk many kilometers to the bottom. The scientists stress that human activities and climate change risks upsetting the valuable and delicate role of the ocean’s surface ecosystem.

Olivier Sulpis, first author of the publication and NESSC-researcher at Utrecht University, underlines the crucial role of calcium carbonate minerals in the ocean. “These minerals are found everywhere: they are used to build the seashells you find on the beach, the shells of microscopic planktonic organisms, they are produced in the stomachs of fish. After ocean lifeforms die, these minerals sink kilometers down to the oceans floor, and either dissolve as they sink down – or become part of the sea floor and turn to stone over time. They are useful for us too, as very old seafloors can become part of mountains, like the Alps or the Himalayas, from which we take the limestone to build beautiful castles and houses. “
Ocean ecosystem
In collaboration with colleagues from NORCE and the Bjerknes Centre for Climate Research in Bergen (Norway) and the University of Bern (Switzerland), the scientists focused on two objectives: to better understand how much of calcium carbonate is actually present in the ocean, and to find out where in the ocean the mineral dissolves. Sulpis: “We find that a lot of calcium carbonate dissolves at the surface of the ocean, particularly in the first kilometre. This is mainly caused by biological processes from all kind of organisms, rather than by chemical processes. Microbes, for instance, eat organic materials and produce CO2 which is then used for dissolving calcium carbonate minerals. It is part of the natural cycle, happening at a tiny scale. However, extra CO2 in the atmosphere likely will influence these processes.”
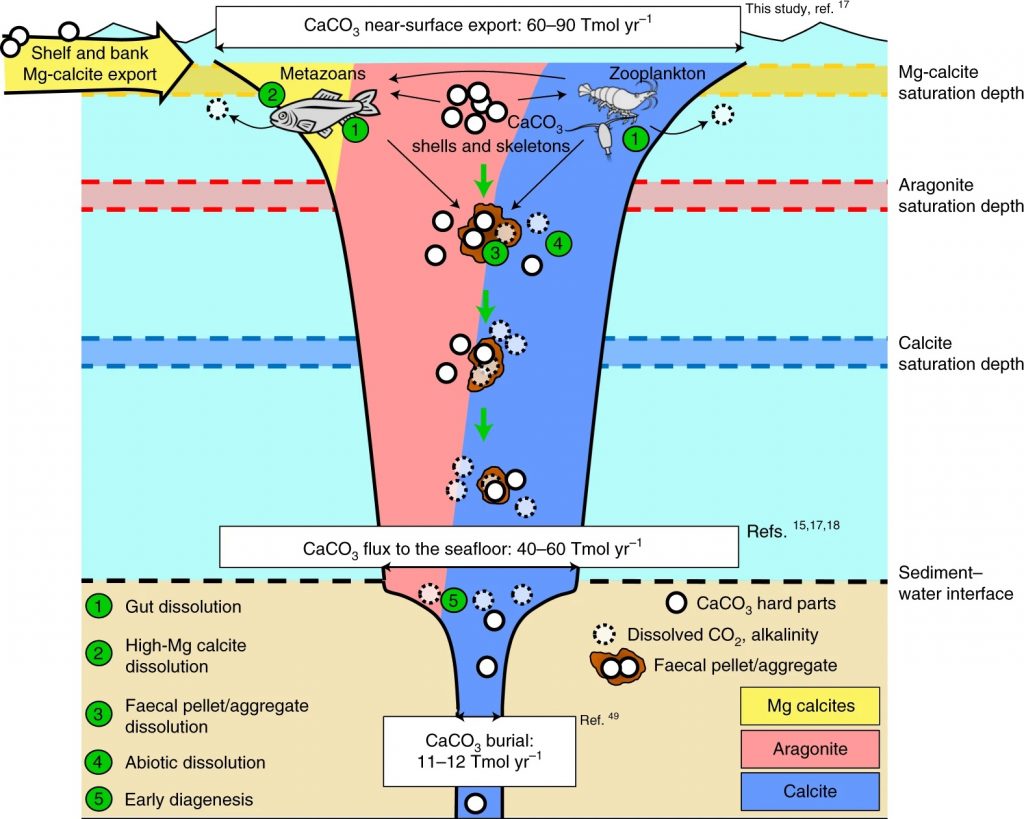
Dissolution at the surface ocean was found to be the largest in particular areas of the oceans: the subtropical gyres. “In these areas you find a lot of single-celled algae, named coccolithophores, that build plates of calcium carbonate, almost like shields. Because these organisms are tiny and very light, when they die, it can take weeks or months before their body arrive at the bottom. This means that the odds of their hard parts being dissolved on the way down are higher,” Sulpis explains.
Predicting consequences
Because calcium carbonates act as anti-acid pills in the ocean ecosystem, it is important to understand how the different factors controlling their dissolution are going to respond under the influence of the increasing CO2 concentrations and warming ocean water. Plus, marine ecosystems are also subject to other consequences of human activities, such as pollution or overfishing. “It’s never easy to make predictions when life is involved,” adds Sulpis. However, too much CO2 can cause problems for organisms in the oceans: calcium carbonate shells become harder to build and easier to dissolve. “It can take decades before we see these processes change, and we haven’t studied the oceans in this detail for that long. But, in general, it’s not a great idea to change a natural ecosystem if you don’t know how it will respond.”
Article:
Calcium carbonate dissolution patterns in the ocean
Nature Geoscience, 2021
Sulpis, O., Jeansson, E., Dinauer, A., Lauvset, S. K., Middelburg, J. J.
doi.org/10.1038/s41561-021-00743-y

